
Dr. Lázaro Eustáquio Pereira Peres
Assistant Professor of Plant Physiology
Universidade de São Paulo (USP)
Escola Superior de Agricultura ¨Luiz de Queiroz¨ (ESALQ)
Departamento de Ciências Biológicas (LCB)
Av. Pádua Dias, 11 CP 09
13418-900 Piracicaba, SP - Brazil
Phone: +55-19-34294052 Fax: +55-19-34348295
e-mail: lazaropp@esalq.usp.br
Educational Background
B.S. in Agronomy, Universidade Federal de Brasilia, Brazil, 1987-1993
Ph.D in Plant Physiology, Universidade de São Paulo, Brazil, 1993-1998
Postdoctoral Research Associate, Universidade de São Paulo, Brazil, 1998-1999
Visiting Professor, Universidade Federal Rural do Rio de Janeiro, Brazil, 1999-2000
Visiting Academic, Lancaster University, UK, 2006-2007
Assistant Professor, Universidade de São Paulo, 2001-present
Teaching
LCB323 Plant Physiology (Undergraduate students - Biology)
LCB311 Plant Physiology (Undergraduate students - Agronomy)
LCB5719 Plant Physiology II - Development (Graduate students)
CEN5763 Plant Physiology and Biotechnology (Graduate students)
Research Interest
Hormones are molecules involved in virtually every step of plant development. Tomato is the model of choice to study developmental processes such as fleshy climacteric fruits, compound leaves, and multicellular trichomes, which are all absent in the model plant Arabidopsis thaliana. Since tomato is a species of major economic importance, it is ideal to bring together basic and applied plant sciences. The tomato Micro-Tom (MT) cultivar makes it possible to combine the direct benefits of studying a crop species with the fast life cycle and small size required for a suitable biological model. In 1998, we started to develop MT mutant and transgenic lines affecting hormone metabolism and signaling (Carvalho et al. Plant Methods, 7:18, 2011), as well as other important molecules controlling plant development (photoreceptors, transcription factors, etc). Such genotype collection is a useful toolkit to discover novel molecular functions and interactions. Since all mutants are in the same genetic background (cv Micro-Tom), comparative studies are made easy and the elegant approach of double mutant analysis can be performed.
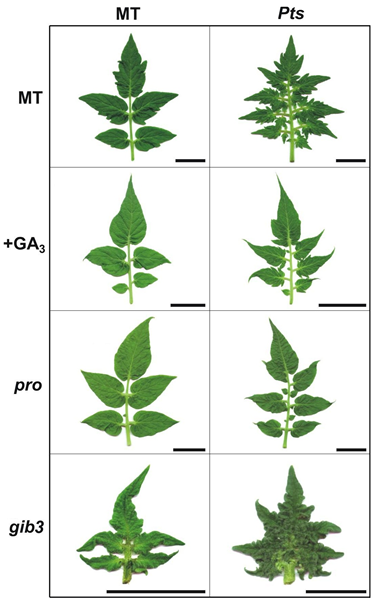
The figure above is an example of double mutant analysis showing the interaction between the KNOX gene Petroselinum (Pts) and the gibberellin (GA) mutants pro (a DELLA mutant) and gib3 (GA-deficient). The Pts allele is a natural genetic variation introgressed (through successive backcrosses) from a wild relative endemic in the Galapagos Islands (Solanum galapagense). The tomato wild relatives evolved in a variety of contrasting environments, so they have unique developmental traits controlled by novel alleles or alleles lost during tomato domestication. Although induced mutagenesis (through EMS and gamma-ray) is a powerful tool to obtain defective alleles (e.g. pro and gib3) in MT, such procedure is unlikely to be a good source of lines harboring functional allelic variation for genes already knocked out. Thus, the introgression of natural genetic variations and wild type alleles complements our program of induced mutagenesis (Pino-Nunes et al. Acta Horticulture, 821:63-72, 2009) and high throughput transgenic plant production (Pino et al. Plant Methods, 6:23, 2010) in MT. The complete list of MT mutants, transgenes and natural genetic variations in our collection is available in the link ¨TOMATO MUTANTS¨. Some of these lines are being used by colleagues and collaborators around the world to study plant development and how plants control it to better explore environmental resources and cope with different types of stress. Examples of overseas collaborators are Dr. Jose Luis Garcia-Martinez (Valencia, Spain), Dr. Francisco Perez-Alfocea (Murcia, Spain), Dr. Ian C. Dodd (Lancaster, UK) and Dr. Vagner A. Benedito (West Virginia, USA). Other main collaborators in Brazil and abroad are listed in the publications below.
24. Campos ML, Carvalho RF, Pino LE, Crestana SL, Zsögön A, Lima JE, Benedito VA, Peres LEP (2011) Convergence of developmental mutants into a single tomato model system: ‘Micro-Tom’ as an effective toolkit for plant development research. Plant Methods, 7:18.
23. Canellas LP, Dantas DJ, Aguiar NO, Peres LEP, Zsögön A, Olivares FL, Dobbss LB, Façanha AR, Nebbioso A, Piccolo A (2011) Probing the hormonal activity of fractionated molecular humic components in tomato auxin mutants. Annals of Applied Biology, in press
22. Carvalho RF, Aidar ST, Azevedo RA, Dodd IC, Peres LEP (2011) Enhanced transpiration rate in the high pigment 1 tomato mutant and its physiological significance. Plant Biology
21. Monteiro CC, Carvalho RF, Gratão PL, Carvalho G, Tezotto T, Medici LO, Peres LEP, Azevedo RA (2011) Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environmental and Experimental Botany, 71:306-320
20. Serrani JC, Carrera E, Ruiz-Rivero O, Gallego-Giraldo L, Peres LEP, Garcia-Martinez JL (2010) Inhibition of auxin transport from the ovary or from the apical shoot induces parthenocarpic fruit-set in tomato mediated by gibberellins. Plant Physiology, 153:851-862.
19. Pino LE, Lombardi-Crestana S, Azevedo MS, Scotton DC, Borgo L, Quecini V, Figueira A, Peres LEP (2010) The Rg1 allele as a valuable tool for genetic transformation of the tomato Micro-Tom model system. Plant Methods, 6:23.
18. Carvalho RF, Quecini V, Peres LEP (2010) Hormonal modulation of photomorphogenesis-controlled anthocyanin accumulation in tomato (Solanum lycopersicum L. cv Micro-Tom) hypocotyls: Physiological and genetic studies. Plant Science, 178:258-264.
17. Campos ML, Carvalho RF, Benedito VA, Peres LEP (2010) Small and remarkable: The Micro-Tom model system as a tool to discover novel hormonal functions and interactions. Plant Signaling & Behavior, 5:267-270.
16. Dobbss LB, Canellas LP, Olivares F, Aguiar NO, Peres LEP, Azevedo M, Spaccini R, Piccolo A, Façanha AR (2010) Bioactivity of chemically transformed humic matter from vermicompost on plant root growth. Journal of Agricultural and Food Chemistry, 58:3681-3688.
15. Lima JE, Benedito VA, Figueira A, Peres LEP (2009) Callus, shoot and hairy root formation in vitro as affected by the sensitivity to auxin and ethylene in tomato mutants. Plant Cell Reports, 28:1169-1177.
14. Campos ML, de Almeida M, Rossi ML, Martinelli AP, Litholdo Jr CG, Figueira A, Rampelotti-Ferreira FT, Vendramim JD, Benedito VA, Peres LEP (2009) Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. Journal of Experimental Botany, 60:4347-4361.
13. Gratão PL, Monteiro CC, Rossi ML, Martinelli AP, Peres LEP, Medici LO, Lea PJ, Azevedo RA (2009) Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environmental and Experimental Botany, 67:387-394.
12. Peres LEP, Zsögön A, Kerbauy GB (2009) Abscisic acid and auxin accumulation in Catasetum fimbriatum roots growing in vitro with high sucrose and mannitol content. Biologia Plantarum, 53:560-564.
11. Aranda-Peres AN, Peres LEP, Higashi EN, Martinelli AP (2009) Adjustment of mineral elements in the culture medium for the micropropagation of three Vriesea bromeliads from the Brazilian Atlantic Forest: the importance of Calcium. HortScience, 44:106-112.
10. Gratão PL, Monteiro CC, Antunes AM, Peres LEP, Azevedo RA (2008) Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium-induced stress. Annals of Applied Biology, 153:321-333.
9. Zsögön A, Lambais MR, Benedito VA, Figueira AV de O, Peres LEP (2008) Reduced arbuscular mycorrhizal colonization in tomato ethylene mutants. Scientia Agricola, 65:259-267.
8. Gratão PL, Monteiro CC, Peres LEP, Azevedo RA (2008) The isolation of antioxidant enzymes from mature tomato (cv. Micro-Tom) plants. HortScience, 43:1608-1610.
7. Dobbss LB, Medici LO, Peres LEP, Pino-Nunes LE, Rumjanek VM, Façanha AR, Canellas LP (2007) Changes in root development of Arabidopsis promoted by organic matter from oxisols. Annals of Applied Biology, 151:199-211.
6. Peres LEP, Carvalho RF, Zsögön A, Bermudez-Zambrano OD, Robles WGR, Tavares S (2005) Grafting of tomato mutants onto potato rootstocks: An approach to study leaf-derived signaling on tuberization. Plant Science, 169:680-688.
5. Lima JE, Carvalho RF, Tulmann Neto A, Figueira A, Peres LEP (2004) Micro-MsK: a tomato genotype with miniature size, short life cycle and improved in vitro shoot regeneration. Plant Science, 167:753 -757.
4. Peres LEP, Morgante PG, Vechi C, Kraus JE, Van Sluys M-A (2001) Shoot regeneration capacity from roots and transgenic hairy roots of different tomato cultivars and wild related species. Plant Cell, Tissue and Organ Culture, 65:37-44.
3. Peres LEP, Amar S, Kerbauy GB, Salatino A, Zaffari GR, Mercier H (1999) Effects of auxin, cytokinin and ethylene treatments on the endogenous ethylene and auxin-to-cytokinin ratio related to direct root tip conversion of Catasetum fimbriatum Lindl. (Orchidaceae) into buds. Journal of Plant Physiology, 155:551-555.
2. Peres LEP, Kerbauy GB (1999) High cytokinin accumulation following root tip excision changes the endogenous auxin-to-cytokinin ratio during root-to-shoot conversion in Catasetum fimbriatum Lindl. (Orchidaceae). Plant Cell Reports, 18:1002-1006.
1. Zaffari GR, Peres LEP, Kerbauy GB (1998) Endogenous levels of cytokinins, IAA, ABA, and pigments in variegated somaclones of micropropagated banana leaves. Journal of Plant Growth Regulation, 17:59-61.




